How the Advancements in Single-Cell Analysis are Driving the Next Wave of Biopharma Innovation
Discover how automated, ultra-high-throughput single-cell analysis platforms accelerate antibody discovery and cell line development.
Author: Katya Mameishvili, PhD
Single-cell analysis has evolved from a niche innovation to a cornerstone of biopharma R&D. Today, it plays a vital role across the drug development pipeline, from early discovery to therapeutic validation. But as drug targets and therapeutic modalities grow more complex, expectations for single-cell platforms are also rising.
Dr Romina Durigon, Senior Field Application Scientist at Sphere Bio, said: “In today’s fast-paced drug discovery landscape, single-cell analysis has become a core technique for uncovering cellular heterogeneity. Yet, many existing platforms struggle to deliver the precision or throughput required for modern workflows. Researchers often need to rely on multiple assays and disconnected technologies to build a complete picture, which adds significant time, cost, and workflow inefficiencies.”
Traditional workflows often lack the precision and functional depth required for modern antibody discovery, functional screening, and cell line development programs. They typically generate limited data per cell and rely on manual steps that introduce variability and slow progress. There is now a growing demand for high-throughput platforms that deliver real-time, functional insights.
“Researchers are seeking versatile technologies that go beyond the classical monoclonal antibody screening,” explains Durigon. “They need systems that support dynamic cellular readouts, like functional screening, and throughput. Ultimately, such platforms improve R&D efficiency and accelerate decision-making.”
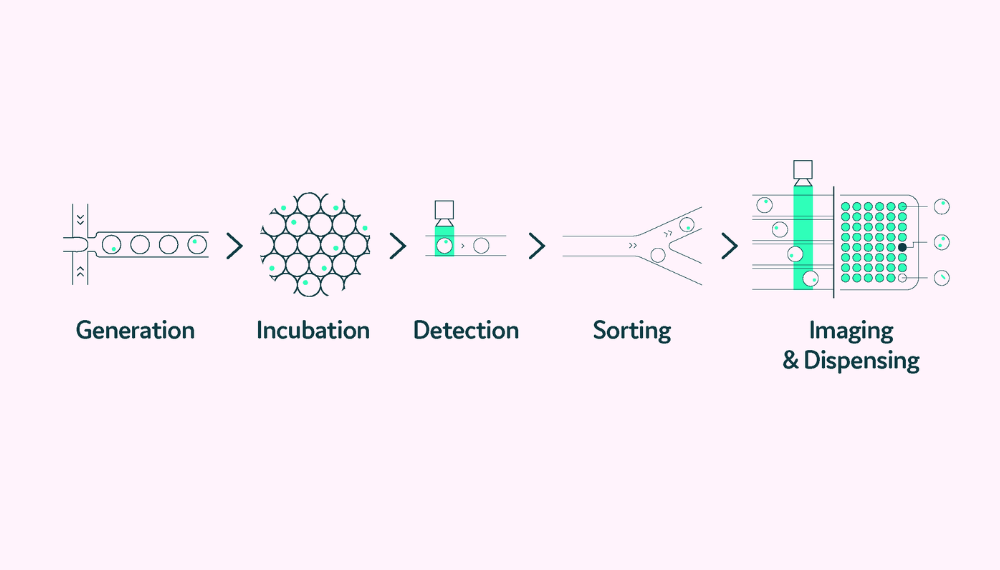
As single-cell analysis technology has evolved from analyzing a few cells to analyzing vast numbers of cells, biopharma scientists have moved beyond examining solely a cell’s genetic makeup to a more functional approach. To meet these evolving needs, next-generation single-cell platforms are integrating precision, scalability, and automation, using technologies like droplet-based microfluidics to increase throughput while conserving precious reagents and samples.
This blog explores how next-generation single-cell platforms are rising to meet these challenges and how they’re transforming antibody discovery, cell line development, and functional screening.
High throughput: From bottleneck to breakthrough
Throughput remains a major bottleneck in many single-cell applications. As researchers hunt for rare, high-value cells within heterogeneous populations, the ability to screen millions to tens of millions of cells daily becomes critical.
Durigon explains: “When you search for very rare cells within a large population, the only way to improve your chances of finding them is to screen more cells. That’s why high-throughput functional screening is crucial, especially when combined with technologies like droplet-based microfluidics.”
Single-cell platforms using droplet-based microfluidics can encapsulate individual cells into tiny, uniform droplets. This miniaturization both conserves the precious samples and reagents and significantly increases throughput. Researchers can analyze millions of cells in parallel, while capturing real-time functional data, such as protein secretion, viability, and activity, all in real time.
This capability accelerates both cell line development and antibody discovery. In cell line development, high-throughput screening allows teams to identify high-producing, viable clones earlier in the process, shaving months off development timelines and reducing costs by minimizing reagent and consumable use. In antibody discovery, these next-generation platforms make it feasible to functionally screen up to tens of millions of cells per day, allowing the identification of rare antibodies.
Multiplexing: Making every cell count more
Modern single-cell analysis technology increasingly depends on multiplexing—the ability to extract multiple types of information from each cell. Multiplexing allows for the simultaneous measurement of several parameters, such as secretion levels, viability, antigen binding, and protein expression, within the same assay. This approach provides a more comprehensive view of cell phenotype and function, which is critical in workflows like antibody screening, functional profiling, and cell line development.
Romina Durigon states: “Multiplexing within single-cell analysis is a method of collecting multiple data sets from a single sample. It significantly increases efficiency and precision while enabling faster identification of promising candidates.”
In antibody discovery, for example, multiplexing capabilities make it possible to assess secretion, antigen-specific binding, and function in parallel. In cell line development, combining productivity markers with viability indicators helps identify candidates that are both high-performing and more likely to maintain that performance during scale-up.
By extracting more functional information from each cell, multiplexing improves the accuracy of candidate selection and helps differentiate promising leads from false positives. This allows researchers to prioritize candidates more effectively and make better-informed decisions throughout the development process.
Automated, easy-to-use platforms for scaling up with confidence
As single-cell workflows become more complex, manual steps increasingly act as bottlenecks, introducing variability, limiting throughput, and extending development timelines. To address these challenges, researchers are adopting automated platforms that integrate critical steps, such as single-cell isolation, incubation, analysis, and dispensing, into one streamlined workflow.
Durigon explains: “Single-cell platforms are evolving toward greater automation and ease of use, which significantly benefits researchers in antibody discovery and cell line development. Automation streamlines processes, improves data quality, and reduces manual intervention, thereby limiting human errors and variability. At the same time, it accelerates workflows, leading to faster, more reliable results.”
Another key trend is the shift toward compact, benchtop systems that are simple to use and integrate into existing workflows.
Durigon adds: “The smaller, the better. These user-friendly platforms eliminate unnecessary operational complexity and the need for larger infrastructure, making advanced single-cell technologies more accessible to a broader range of scientists and therapeutic applications.”
The combination of throughput, automation, and usability drives the adoption of single-cell analysis beyond core facilities and into mainstream discovery pipelines. Fully integrated systems can also be embedded into larger robotic workstations, allowing labs to scale up operations without adding manual overhead.
Automated, easy-to-use, high-throughput platforms support the growing demand for more complex, higher-throughput screens, enabling faster transitions from screening to candidate selection, while improving reproducibility and statistical confidence.
By reducing hands-on time and standardizing workflows, they help researchers scale with confidence and keep pace with increasingly ambitious discovery goals.
More efficient antibody discovery
Identifying high-value B cells remains a key challenge in antibody discovery. These cells are often rare, and traditional methods struggle to isolate and characterize them efficiently. Next-generation single-cell platforms improve this process by enabling high-throughput, antigen-specific screening while maintaining cell viability.
Multiplexing capabilities add further value, allowing selection based on multiple traits, such as secretion level, isotype, and cell viability, within the same workflow. This leads to faster, better-informed decisions and ultimately improves the quality and speed of candidate selection.
Accelerated cell line development
Cell line development is foundational for biologics manufacturing, where selecting high-producing, stable, and viable clones early is critical. Next-generation single-cell analysis platforms accelerate this process through automated, high-throughput screening and integrated functional analysis.
Combining high throughput with functional screening in droplet-based microfluidics has been demonstrated to be particularly effective in the cell line development workflow.
“Researchers can now upgrade the screen for high-producing viable clones early in the development process,” says Durigon. “This approach enhances the efficiency of the cell line selection, increases productivity of final growth, reduces project timelines by up to three months, and saves significant costs due to the lower reagent and consumable use.”
These platforms also support standard regulatory requirements such as monoclonality assurance through built-in imaging and tracking that provide visual confirmation of single-cell origin where needed. This single-cell traceability gives confidence in clone selection and helps meet regulatory expectations, while keeping the focus on performance and scalability.
Functional screening with real-world complexity
Traditional screening often relies on single-parameter readouts, such as expression level or viability. But in complex systems like immune and tumor cell populations, this narrow view is no longer sufficient.
Next-generation single-cell platforms enable multi-parameter functional screening, combining secretion profiles, surface marker expression, and viability data from each cell. This allows for more nuanced candidate selection, better characterization of cellular heterogeneity, and more informed decision-making strategies based on real functional capacity.
Durigon explains: “Single-cell functional screening is now considered essential for early-stage research. It provides a more comprehensive view of cellular state and response to stimuli, which is critical for guiding discovery.”
As demand grows for deeper, more versatile screening, platforms must be able to handle large numbers of cells and perform complex multiplexing assays. Researchers increasingly seek to measure multiple analytes—genomic, transcriptomic, proteomic, and even metabolomic— within a single cell or a group of cells. To meet this need, next-generation single-cell platforms are evolving to deliver both ultra-high-throughput and multiplex capabilities.
The future of single-cell workflows
The next wave of therapeutic innovation demands deeper, more functional insights into individual cells. Research areas like immuno-oncology and cell and gene therapy require granular insight into cellular functions, heterogeneity, and response dynamics, making them a natural fit for next-generation single-cell platforms.
By combining ultra-high-throughput, multiplexing, and ease of use, advanced single-cell platforms are redefining what’s possible in antibody discovery and cell line development.
When integrated early in R&D, these platforms accelerate timelines, improve data quality, and reduce downstream risks, providing a stronger foundation for critical decisions. They can also generate vast multidimensional data sets, ideal for AI and machine learning models, enabling the discovery of novel biomarkers, novel therapeutic targets, and predictive signatures.
“In the years ahead, single-cell platforms will not just be experimental tools, but will also be the bedrock of AI-driven discovery workflows,” says Durigon. “Forward-looking organizations that invest now in these capabilities will unlock new efficiencies and maintain a leadership edge in a rapidly evolving industry.”

Sphere Bio is helping lead this shift with technologies like Cyto-Mine® Chroma, designed to meet the real-world demands of modern therapeutic development. As the field advances, single-cell platforms capable of delivering high-quality, high-throughput insights will form the backbone of discovery workflows across the biopharma landscape.
Explore how Cyto-Mine® Chroma is redefining scalable, multiplex single-cell screening.
The Next Generation: Cyto-Mine® Chroma
Automate. Accelerate. Analyze millions of cells in a single day.
Think Cyto-Mine®, but supercharged, enabling multiplexing and greater assay flexibility to fit your needs. It means that you can examine vastly greater numbers of cells — and isolate the most valuable ones— with unparalleled precision.






